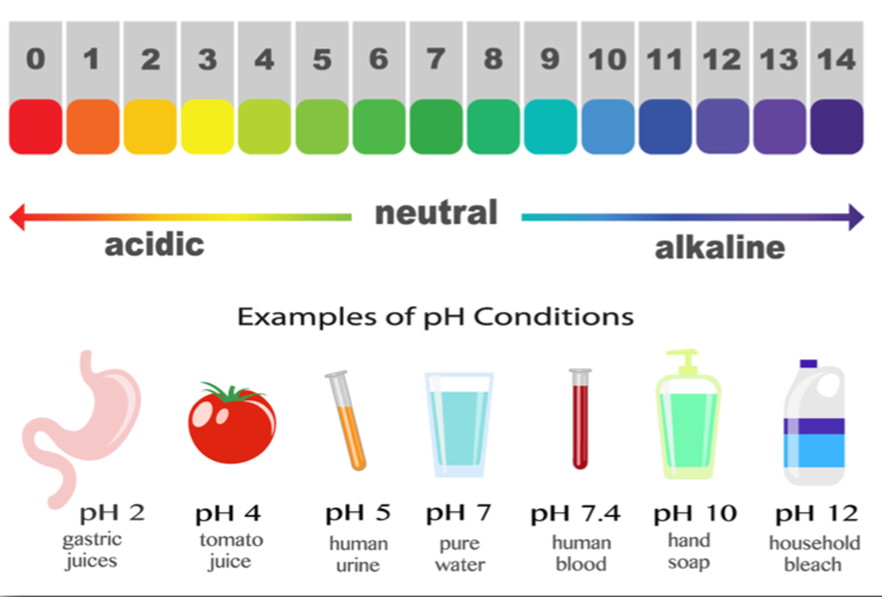
A century ago, chemist Søren Sørensen invented what would become a crucial diagnostic tool: the pH scale.
Since its founding in 1876 by beer magnate J.C. Jacobsen, the Carlsberg Laboratory in Copenhagen has been a center of biochemical discovery. At the turn of the twentieth century, its scientists synthesized several of the amino acids essential to human health and analyzed the chemistry of proteins. And exactly 100 years ago, Søren Sørensen, Carlsberg’s director of chemistry, devised a vital diagnostic tool for measuring acidity, thus advancing the detection of digestive, respiratory and metabolic disorders.
During the 1890s, the Latvian chemist Wilhelm Ostwald and others had invented electrical conductivity equipment that could measure the quantity of hydrogen ions in a solution, but Sørensen succeeded in expressing those measurements in an elegant formula and placing the results on a simple scale. Søren Sørensen developed the pH scale in 1909.The solutions he tested received pH values running from 0 (the most acidic) to 14 (the most alkaline).pH is also known as ‘Potential of Hydrogen’ or ‘Power of Hydrogen’

pH is a measure of the amount of Hydrogen ions (H+) in a solution. … Even in pure water ions tend to form due to random processes (producing some H+ and OH- ions). The amount of H+ that is made in pure water is about equal to a pH of 7. That’s why 7 is neutral.
pH plays a very important role in our everyday life. In our digestive system: Hydrochloric acid produced in our stomach helps the digestion of food without causing any harm to the stomach. But when the amount of the acid goes beyond a certain limit due to indigestion, pain and irritation are created in the stomach.
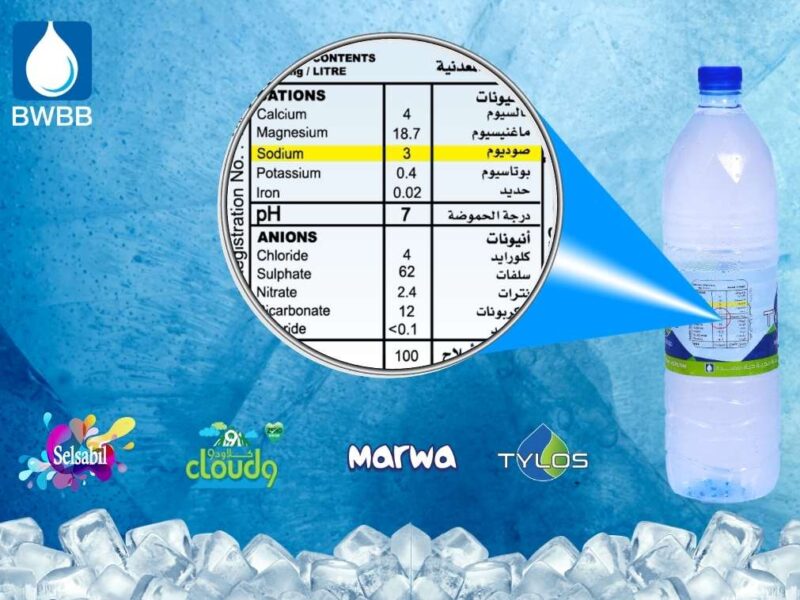
We at BWBB maintain the pH level is 7 – 7.5 to give the exact level which is required human body. Also this will help to balance the blood pH level and reduce from dehydration.